- +91 9403890582
- [email protected]
- Mon - Fri: 9:30 - 18:30
Master Clinical Research, CDM, PV & Clinical SAS – All in One Powerful Online Program

“We enable our students to be job-ready by providing the most updated PGDCR + Clinical SAS training content, insightful delivery, and an innovative learning approach at competitive prices”
Unlock your career in global clinical research with BCRI’s 7.5-month advanced diploma program. This live online course equips you with in-demand skills across Clinical Research, Clinical Data Management, Pharmacovigilance, and Clinical SAS Programming, preparing you for top roles in CROs, Pharma companies, and global sponsors.
Join a Job oriented Training at BCRI, with Excellent Placement Track Record.
BCRI’s PG Diploma in Clinical Research with Clinical SAS Programming is a 7.5-month live online training program designed to make you industry-ready across Clinical Research, Clinical Data Management, Pharmacovigilance, and Clinical SAS Programming.
Join a job-orientated PG Diploma in Clinical Research with SAS training at BCRI, with an excellent placement track record.
Our 7.5-month training curriculum is designed to equip you with industry-ready knowledge across four key domains: Clinical Research, Clinical Data Management, Pharmacovigilance, and Clinical SAS Programming. Each module is taught with real-world case studies, tools, and hands-on assignments — preparing you for a successful career in clinical trials, regulatory submissions, and biostatistics.
Covers Clinical Research, CDM, Pharmacovigilance & Clinical SAS Programming in a structured, progressive format.
Learn from senior professionals with 10–15+ years of experience in CROs, Pharma companies & Clinical SAS domains.
Get practical exposure in Base & Advanced SAS, CDISC SDTM, ADaM & TLFs — aligned with real clinical trial data standards.
Apply your learning through an optional real-site internship for exposure to live trial environments.
Prepare for global roles like Clinical SAS Programmer, Data Analyst, CRA, Drug Safety Associate & more.
Revisit your sessions anytime, anywhere with BCRI’s learning app for unlimited revisions.
Resume prep, mock interviews & interview referrals to CROs, hospitals, and pharma analytics firms.
Curriculum aligned with ICH-GCP, FDA, CDISC, MedDRA, and pharmacovigilance standards used globally.
Flexible Learning, Career-Focused Training
| Feature | Details |
|---|---|
| Course Name | PG Diploma in Clinical Research with Clinical SAS Programming |
| Duration | 7.5 Months (Live Online Classes) |
| Mode of Delivery | Live Instructor-Led Online Sessions (Includes recordings, notes, and LMS access) |
| Focus Area | Clinical Research, Clinical Data Management, Pharmacovigilance, and Clinical SAS Programming |
| Key Topics Covered | Base SAS, Advanced SAS, CDISC SDTM, ADaM, TLFs, GCP, CRF Design, AE/SAE Reporting, Data Validation |
| Tools/Formats Used | SAS (Base & Advanced), CDISC, SDTM, ADaM, MedDRA, eCRF systems |
| Eligibility | B.Pharm, M.Pharm, B.Sc, M.Sc (Life Sciences), MBBS, BDS, BHMS, BAMS, or working professionals in healthcare |
| Assessment | Assignment-based evaluation + Mock tests + Final Assessment + Project-based evaluation |
| Certificate | BCRI PG Diploma Certificate + Certification in Clinical SAS (on completion) |
| Placement Support | 100% Placement Assistance – Resume Building, Mock Interviews, Interview Referrals, Internship with SMOs/CROs |
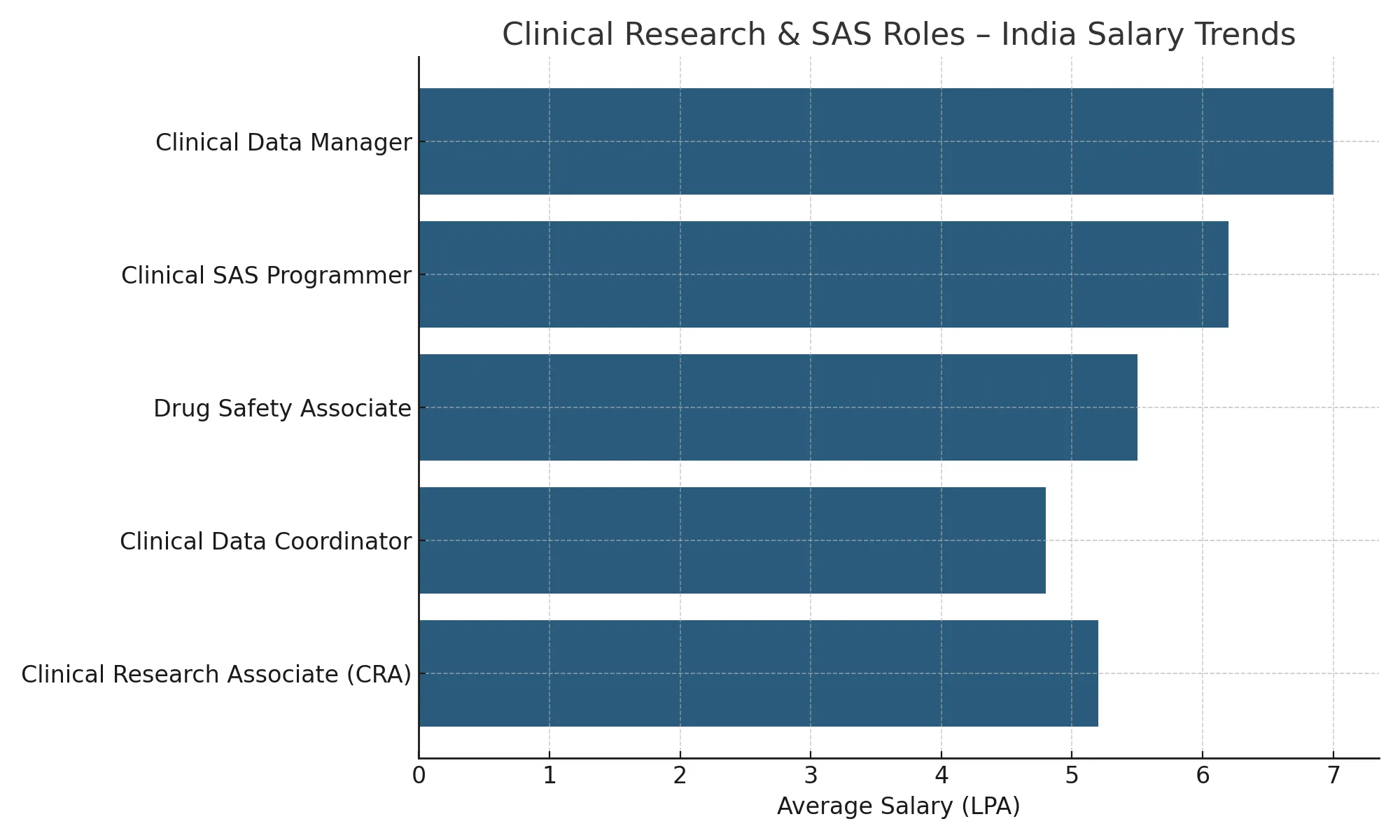
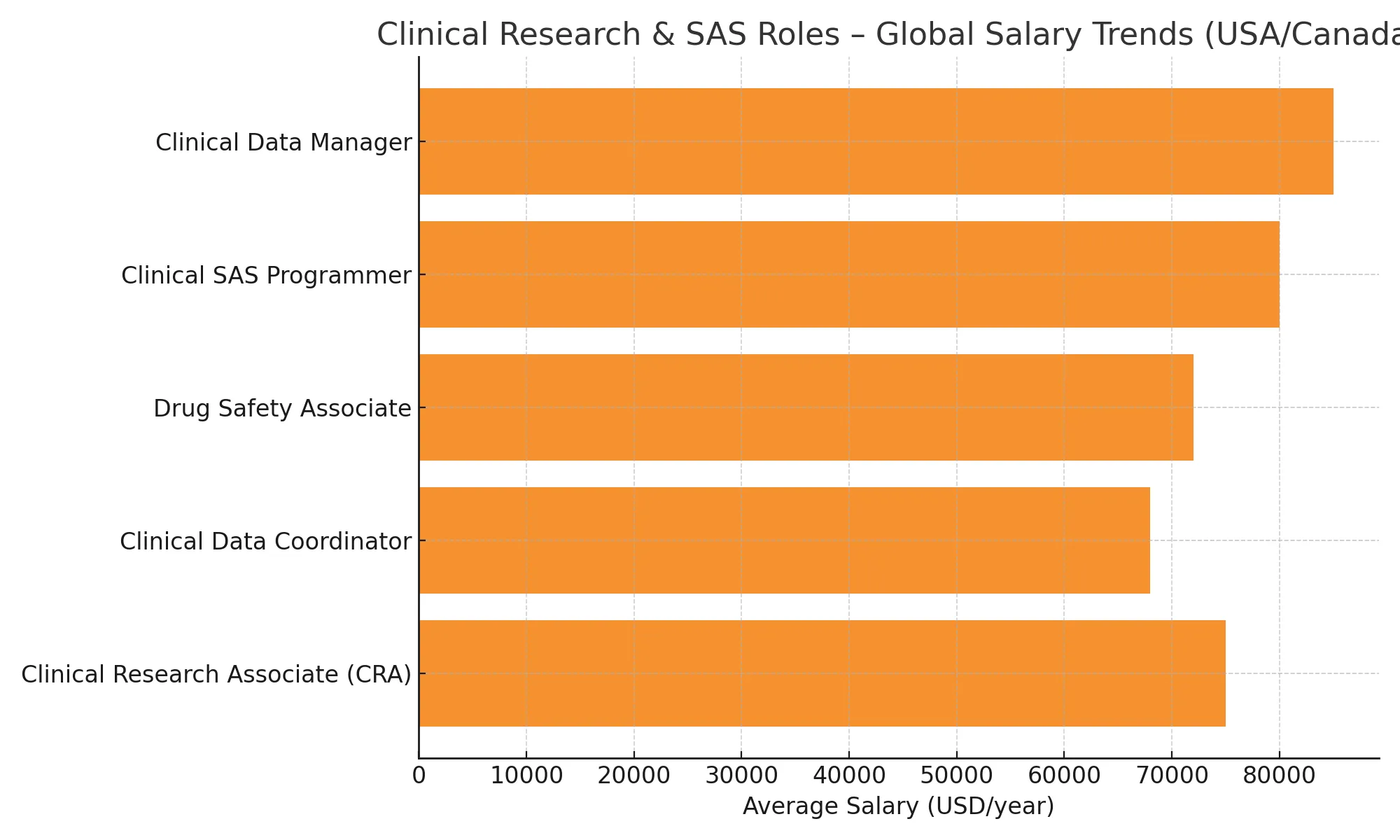
Graduates of BCRI’s PG Diploma in Clinical Research with Clinical SAS Programming are eligible for multiple high-demand roles across Clinical Data Management, Pharmacovigilance, and SAS Programming. Below is a comparative view of salary expectations across key global markets. These trends are derived from authentic sources including Payscale, AmbitionBox, Glassdoor, and official government labor statistics.
Salaries in the Clinical Research domain vary based on geography, job role, and experience. Below are indicative salary ranges across India, USA, and Canada.
| Job Role | India (₹/Year) | USA ($/Year) | Canada (C$/Year) |
|---|---|---|---|
| Clinical Data Manager | ₹5–8 LPA | $85,000–115,000 | C$70,000–90,000 |
| Pharmacovigilance Associate | ₹4.5–7.5 LPA | $75,000–100,000 | C$65,000–85,000 |
| Clinical SAS Programmer | ₹6–10 LPA | $90,000–125,000 | C$75,000–95,000 |
| Clinical Research Associate | ₹4.5–8.5 LPA | $70,000–110,000 | C$65,000–90,000 |
Take the next step in your career with our industry-recognized PG Diploma in Clinical Research. Secure your spot today!
The eligibility criteria for the Post Graduate Diploma in Clinical Research (PGDCR) program are that the Candidates should have completed their graduation degree in any of the following fields: a) Science (e.g., Biochemistry, Microbiology, Genetics, Biotechnology, Botany, Zoology) b) Medicine (e.g., MBBS, BDS, BPT, BAMS, BHMS, BUMS) c) Pharmacy or Pharmaceutical Sciences(e.g B.Pharm, Pharm.D).
After completing a PG Diploma in Clinical Research, graduates can work in various roles such as Clinical Research Associate, Clinical Data Manager, Biostatistician, Clinical SAS Programmer, Pharmacovigilance Specialist, and many more in pharmaceutical and healthcare companies.
Our 7.5-month program PG diploma in clinical research covers major domains like Clinical Research, Clinical Data Management, Pharmacovigilance, Regulatory Affairs, and Clinical SAS. Students benefit from practical, hands-on training and gain expertise in using EDC software like Clinevo and Saftey Database—a valuable advantage for our students!
The PG Diploma in Clinical Research programme at BCRI includes domains like Clinical Research (CR), Clinical Data Management (CDM), Clinical SAS (SAS), Regulatory Affairs (RA), Pharmacovigilance and Drug Safety (PV), Professional and Career Development (PCD), Practical Training and Internship (PTI).
Certainly! Completing the Post Graduate Diploma in Clinical Research (PGDCR) equips graduates with a robust set of practical skills and competencies that enhance their employability in the clinical research job market. Here are the key areas where PGDCR graduates with BCRI benefits, students learn to handle patient recruitment, data collection, adverse event monitoring, and protocol adherence. Understanding regulatory guidelines is crucial. PGDCR covers topics related to Good Clinical Practice (GCP), ICH guidelines, and local regulations. PGDCR imparts skills in clinical data management, including data entry, validation, and quality control, and learns to use statistical tools for data analysis.
Certainly! In a Post Graduate Diploma in Clinical Research (PGDCR) program, students typically gain hands-on experience through various avenues: like Electronic Data Base Capture (EDC) in clinical data management, Safety Data Base in pharmacovigilance, Case processing, Narrative writing, Statistical Analysis Software (SAS) etc..
Yes, on Successful completion of PG diploma in Clinical Research course, we do have 100% placement Support on successful completion of the course. Each student is entitled to placement assistance. Our placement officer is dedicated to finding a suitable opportunity for each student. We are proud to state within a short duration we have been successful in placing our students in the field of clinical operations as CRCs and process coordinators, as drug safety associates in Pharmacovigilance departments, QA personnel in medical writing departments and as Jr. SAS programmers.
Students who have completed a course in clinical research from any other organization may approach us for internship assistance.
We cannot guarantee that a company will hire you, as it is based on your performance during an interview. However, we do provide 100% placement assistance. till date, we have 100% Placement record.
The duration of the Post Graduate Diploma in Clinical Research (PGDCR) program typically is 7.5 months. However,this may vary slightly depending on the add on. During this period, students gain in-depth knowledge about clinical research methodologies, regulations, and hands on experience. PGDCR a valuable program for those interested in pursuing a career in the clinical research industry.
Trainers with extensive experience and expertise in the field of clinical research contribute significantly to the quality of education. We have trainers with high industry experience and research backgrounds. A well-structured curriculum that covers essential topics in clinical research, including regulatory, data management, and practical skills, is a hallmark of our reputable institution. BCRI organizations often incorporate hands-on training, practical exercises, and internship opportunities to ensure that students gain real-world experience in clinical research settings.
A student with a Postgraduate Diploma in Clinical Research (PGDCR) can explore a variety of job roles within the clinical research industry as Clinical Research Associate (CRA), Clinical Research Coordinator (CRC), Clinical Data Manager (CDM), SAS Programmer, Clinical Trial Manager (CTM), Drug Safety Associate (DSA), Regulatory Affairs Specialist, Medical writer, Site Management Organization (SMO) Coordinator etc.

Real success stories from our students who have transformed their careers with our PG Diploma in Clinical Research (PGDCR) Training! Watch her testimonials and hear firsthand how our program has helped her secure rewarding opportunity in clinical research, pharmacovigilance, and regulatory affairs.
📢 Why Our Students Love BCRI:
✔ Career-changing learning experience
✔ Expert-led training with real-world case studies
✔ Strong industry connections and placement support
👉 Watch her Story ..
Address: 19/19 Chandrodaya Complex, 2nd Floor, 24th Main, Agara Sector 1, HSR Layout, Bangalore, KA 560102, India.
Ph: +91 9403890582
Email: [email protected]
Mon to Fri: 9.00 AM – 6.30 PM
WhatsApp us
Our Team would love to Guide you to select the right course here .